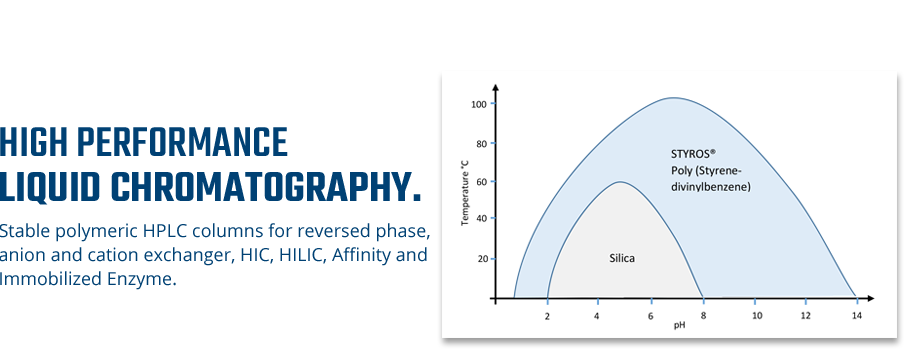
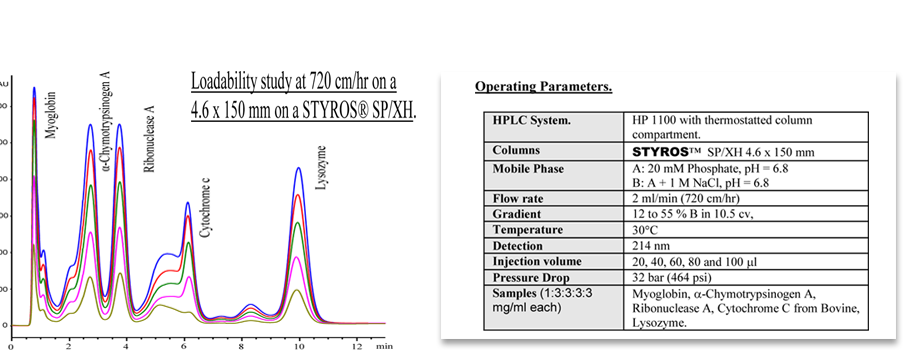
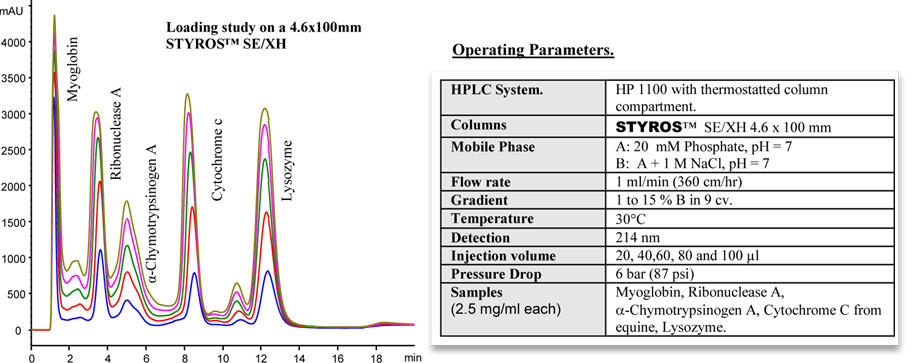
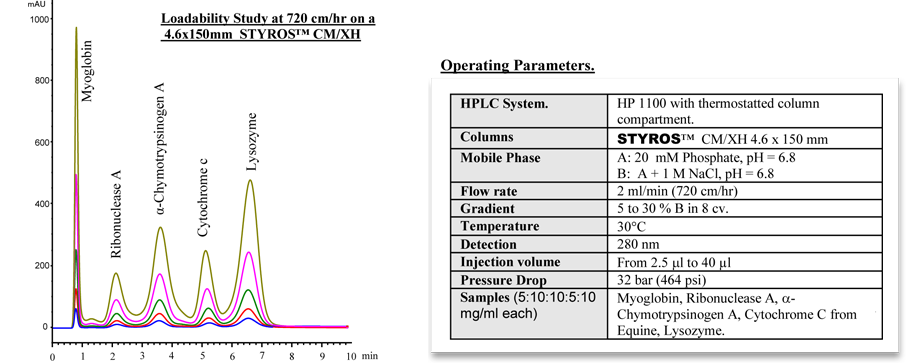
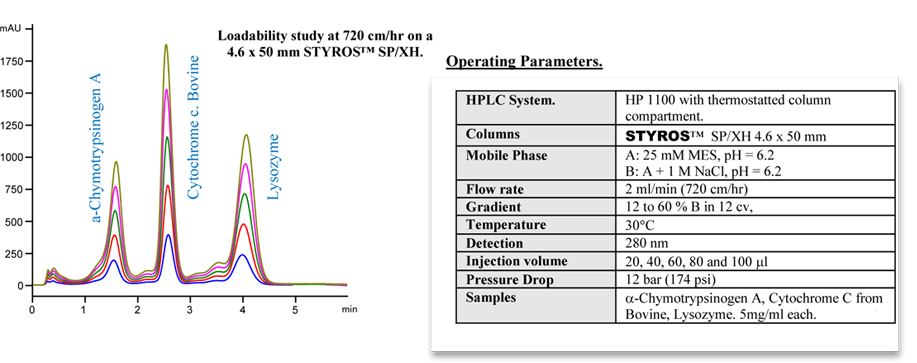
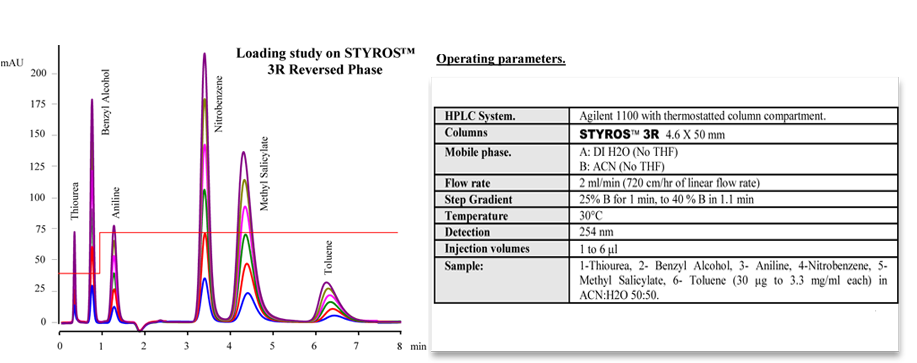
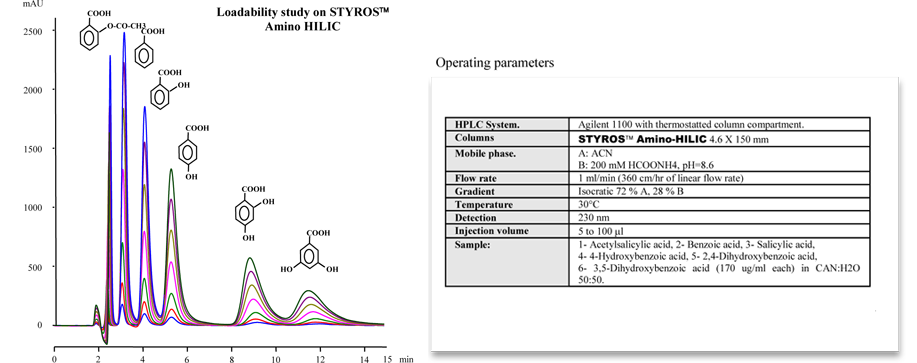
Considering chromatography as the single most important technique for purifying high-value biopharmaceuticals, we thrive in providing the most efficient set of stationary phases based on non leaching and stable Poly (Styrene-divinylbenzene).
Facts & News
Thanks to you, our loyal and ever-growing customers, we have done it for over 25 consecutive years.
Price comparison between STYROS® chromatography columns and Mono Bead packed columns from GE Healthcare (former Pharmacia, Amershame, Pfizer and now Cytiva ).
The focus has always been the performance and stability. The universal leaching of soft gel used predominantly in industry are making it impossible to deliver pure products in a short time as soft gel do not lend itself to automation as hard gel does.